On August 10th, the pioneering team led by Professor He Zhengguo from the School of Life Science and Technology at Guangxi University, in collaboration with the State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources, achieved a groundbreaking advancement in the realm of bacterial virus infections and host defense mechanisms. Their latest research, titled “Mycobacterial phage TM4 requires a eukaryotic-like Ser/Thr protein kinase to silence and escape anti-phage immunity,” has been published online in the esteemed subjournal Cell Host & Microbe of the Cell journal. Professor He Zhengguo is the corresponding author of the paper, and Dr. Xiaohui Li, a postdoctoral researcher, is the first author. Notably, Guangxi University stands as the exclusive institution responsible for realizing this remarkable research milestone.
Representing a significant class within the Actinobacteria group, Mycobacteria, most notably Mycobacterium tuberculosis responsible for human tuberculosis, carries substantial global health implications with an annual toll of nearly 1.3 million fatalities. Notably, the escalating drug resistance in tuberculosis poses a formidable challenge to current prevention and control efforts, necessitating the imperative development of innovative strategies. Bacteriophages, a specialized class of viruses that exclusively prey upon bacteria, emerge as natural adversaries to bacterial populations, displaying robust bactericidal activity and synergizing with conventional antimicrobial agents. Clinical trials have already demonstrated promising efficacy of mycobacteriophages in treating drug-resistant infections, illuminating a promising therapeutic horizon. However, akin to antibiotics, mycobacteriophage therapies also confront issues of tolerance, underscoring the urgency to fortify research into the intricate dynamics of mycobacteriophage-host-bacteria interactions and the underlying mechanisms governing mycobacteriophage resistance. A comprehensive investigation into these fundamental scientific queries is paramount to surmounting the technical barriers in mycobacteriophage therapy.
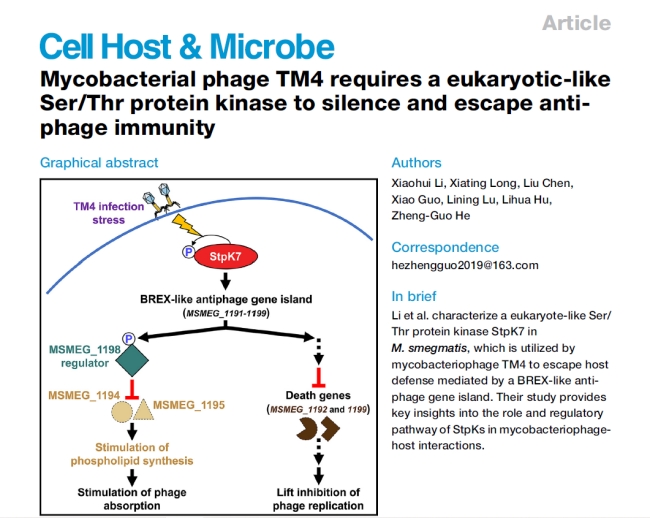
Existing research has already demonstrated the widespread presence of Serine/Threonine protein kinases (StpK) in both eukaryotes and bacteria, typically exerting antiviral effects. Professor He Zhengguo’s team has recently made a significant advancement in this domain by uncovering how the mycobacteriophage TM4, specifically targeting mycobacteria, ingeniously harnesses the bacterial StpK to elude the host’s defense mechanisms. Consequently, this pioneering study not only establishes the existence of the first-ever mycobacteriophage host factor protein kinase but also identifies a gene island regulated by this class of kinase, orchestrating an intricate anti-phage defense mechanism within mycobacteria.
The research notably highlights that during the process of mycobacteriophage infection, TM4 distinctly induces the expression and phosphorylation of intracellular StpK7 within the host mycobacteria. The activated StpK7 serves a dual role. Firstly, it phosphorylates a transcription factor within a defense island reminiscent of BREX, effectively suppressing the downstream expression of target genes. This molecular cascade ultimately promotes phospholipid synthesis and enhances mycobacteriophage adsorption. Simultaneously, StpK7 inhibits the expression of bacterial cell death genes, consequently curbing host bacterial abortive infections. The concurrent action of these two facets notably synergizes to enhance mycobacteriophage infectivity and replication capacity. These findings collectively unveil a distinctive and intricate defense-counterdefense interplay mechanism between mycobacteriophages and mycobacteria, thus offering invaluable insights and novel perspectives for harnessing mycobacteriophages in combatting infectious diseases.
It is worth noting that Professor He Zhengguo joined Guangxi University on a full-time basis in 2019. In 2020, he successfully led a project, with GXU as the lead institution, securing a grant of 19.18 million RMB under the National Key Research and Development Program. In 2022, he obtained funding for another significant project through the National Natural Science Foundation of China. In recent years, Professor He has prominently emerged as the primary author of numerous high-impact research papers published in renowned journals, including Cell Host & Microbe (2023) and Nature Communications (2022). Demonstrating his dedication to education and research, he guided GXU’s undergraduate students to achieve a groundbreaking award at the International Genetically Engineered Machine (iGEM) competition. Furthermore, Professor He has taken the lead in organizing and hosting two major international and domestic academic conferences. Currently, he holds esteemed positions as a council member in both the China Biotechnology Society and the China Society for Microbiology, concurrently serving as Deputy Chair for two specialized committees. The research has received substantial support from both the National Key Research and Development Program and the National Natural Science Foundation of China.
 ???????????????????
>
Content
???????????????????
>
Content
 ???????????????????
>
Content
???????????????????
>
Content




